Home >
News > A New, Methane Adsorbent, Porous Coordination Polymer [CuSiF₆(4,4-bipyridine)₂]
A New, Methane Adsorbent, Porous Coordination Polymer [CuSiF₆(4,4-bipyridine)₂]
Summary:
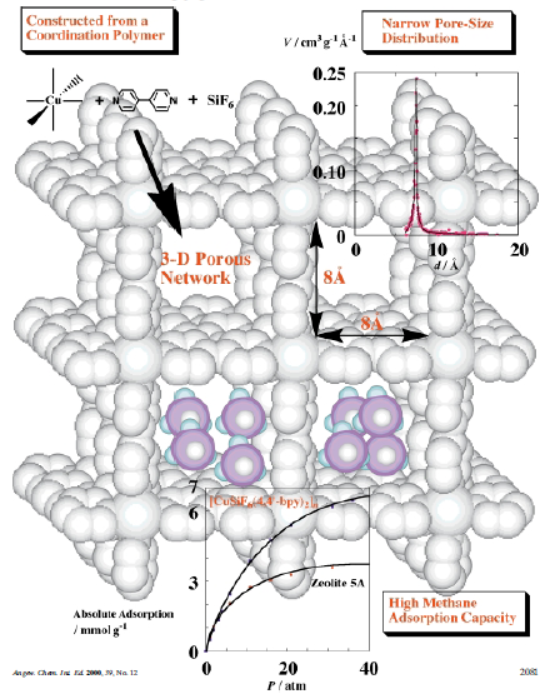
The authors from Kyoto University, Tokyo Metropolitan University, and Osaka Gas Company, Ltd. (Japan) developed a porous coordination polymer {[CuSiF₆(4,4'-bpy)₂]·8H₂O}ₙ (1) with high porosity, narrow pore-size distribution, and robust 3D framework characteristics, achieving excellent methane adsorption performance in the natural gas storage and transport application field.
Background:
1. To address the demand for efficient adsorbents for natural gas (methane) storage and transport, previous researchers focused on porous inorganic materials like zeolites, aluminum phosphates, and their derivatives (studied in detail) but lacked the flexibility of organic-inorganic hybrids. Existing coordination polymers (transition metal ions + organic ligands) often had insufficiently large usable pores, unstable frameworks (wide pressure range at ambient temperature), and synthesis challenges (counteranion channel occupation, interpenetrating networks, framework disruption after guest molecule removal).
2. The authors proposed a rational synthesis strategy: selecting Cu²⁺ (prone to Jahn-Teller distortion, forming weak axial coordination sites) and SiF₆²⁻ (easier to coordinate with Cu²⁺ than solvents, linking copper-containing layers) as building blocks, and 4,4'-bipyridine (4,4'-bpy) as a bridging ligand. This successfully prepared a 3D porous coordination polymer with no interpenetration, stable framework, and large usable channels.
Research Content:
1. Synthesis
The authors synthesized single crystals of material 1 via diffusion: in a straight-type glass tube at room temperature, an aqueous ethylene glycol (1:3) solution containing Cu(BF₄)₂·6H₂O (1.00 mmol) and (NH₄)₂SiF₆ (1.00 mmol) was diffused into an ethylene glycol solution of 4,4'-bpy (2.00 mmol). After one week, purple block-shaped crystals precipitated; 4 water molecules per unit cell evaporated in air to form a partly dehydrated product (yield: 88%). A similar Zn²⁺-based polymer {Zn(4,4'-bpy)₂(SiF₆)}ₙ (2) was synthesized using dimethylformamide/dioxane (5:2) as solvent, and a Cu²⁺-based polymer with GeF₆²⁻ (instead of SiF₆²⁻) was prepared under the same conditions as 1.
2. Characterizations
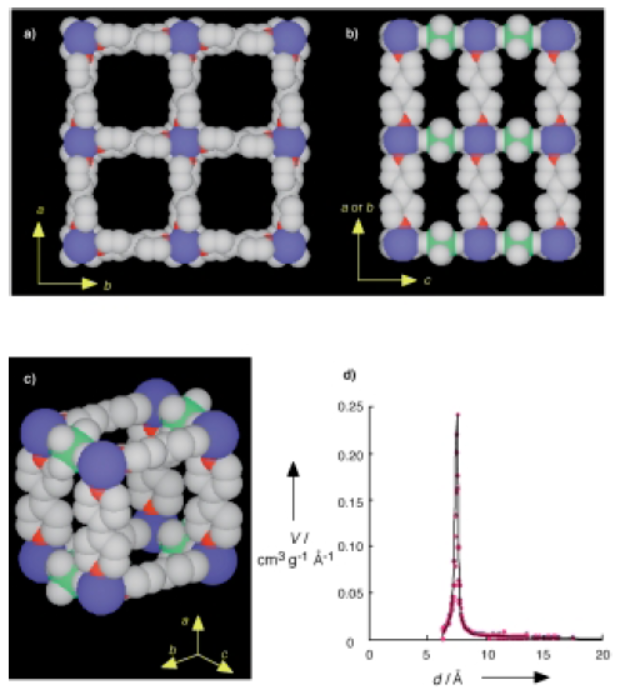
1) BET and pore size distribution: Calculated from argon adsorption at 87.3K (Micromeritics ASAP 2001M), the specific surface area of 1 is 1337 m²g⁻¹, micropore volume is 0.56 mLg⁻¹; Horvath-Kawazoe (HK) plot shows a single sharp peak at ~7.8 Å, confirming narrow pore-size distribution and matching the 8×8 Ų square channels (along c-axis) from X-ray analysis. Argon cannot penetrate 6×2 Ų rectangular channels (along a/b axes) due to size mismatch.
2) XRPD and TG: X-ray powder diffraction (XRPD) shows good agreement between the simulated pattern (single-crystal data without crystallized water) and observed pattern (373 K, ~0.1 mmHg), proving framework stability without guest molecules. Thermal gravimetric (TG) analysis shows crystallized water release below 373 K, framework decomposition at 423 K.
3) X-ray single-crystal diffraction: Confirms 1 has a tetragonal structure (space group P4/mm, a=11.108(1) Å, c=8.1107(9) Å, V=1000.8(1) ų, Z=1); 3D network consists of 2D square grids of {Cu(4,4'-bpy)₂}ₙ (bridged by 4,4'-bpy) and SiF₆²⁻ pillars (no interpenetration); each Cu²⁺ has a (4+2) coordination environment (4 N from 4,4'-bpy, 2 F from SiF₆²⁻), with bond lengths Cu-F=2.355(5) Å and Cu-N=2.011(5) Å.
4) IR and elemental analysis: IR (KBr pellet) has characteristic peaks at ṽ=3412 (br), 3098(w), 3045(w), 1610(m), etc.; elemental analysis of partly dehydrated 1 ({[CuSiF₆(4,4'-bpy)₂]·4H₂O}ₙ) gives found values (C:40.81%, H:3.50%, N:9.27%) close to calculated values (C:40.71%, H:4.10%, N:9.50%).
3. Application
Methane adsorption tests (298 K, 0-36 atm) on 1 and zeolite 5A (benchmark) showed:
- At 36 atm, 1 adsorbs ~6.5 mmol/g methane, higher than zeolite 5A (~3.7 mmol/g).
- Methane density in 1’s micropores (0.21 g/mL at 298 K, 36 atm) is comparable to compressed methane (0.16 g/mL at 300 K, 280 atm), showing strong micropore filling.
- Desorption/readsorption isotherms overlap, confirming framework stability during cycles.
- Langmuir plot gives inherent micropore capacity W₁=10.0 mmol/g; isotherm fits extended Dubinin-Radushkevich (DR) equation (βE₀=8 kJ/mol, P₀q=284 atm).
4. Mechanism
- Framework stability: SiF₆²⁻ coordinates with Cu²⁺ (axial sites from Jahn-Teller distortion), linking 2D {Cu(4,4'-bpy)₂}ₙ grids into 3D network, avoiding interpenetration and counteranion occupation, ensuring stability after water removal.
- Methane adsorption: Narrow pore-size distribution (8×8 Ų channels) and high surface area/volume provide abundant sites; micropore structure enables strong interactions with methane, leading to high adsorption capacity.
Outlook:
This research synthesized {[CuSiF₆(4,4'-bpy)₂]·8H₂O}ₙ with high methane adsorption, stable framework, and narrow pore distribution, addressing limitations of existing coordination polymers and outperforming zeolite 5A. It provides a new adsorbent for natural gas storage, establishes a rational synthesis strategy for organic-inorganic hybrid porous materials, and opens new directions for coordination polymer-based adsorbents.
A New, Methane Adsorbent, Porous Coordination Polymer [CuSiF₆(4,4-bipyridine)₂]
Authors: Shin-ichiro Noro, Susumu Kitagawa, Mitsuru Kondo, Kenji Seki
DOI: 10.1002/1521-3773(20000616)39:12<2081::AID-ANIE2081>3.0.CO;2-A
Link: https://onlinelibrary.wiley.com/doi/10.1002/1521-3773(20000616)39:12<2081::AID-ANIE2081>3.0.CO;2-A
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

