Home >
News > Porous materials with optimal adsorption thermodynamics and kinetics for CO₂ separation
Porous materials with optimal adsorption thermodynamics and kinetics for CO₂ separation
Summary:
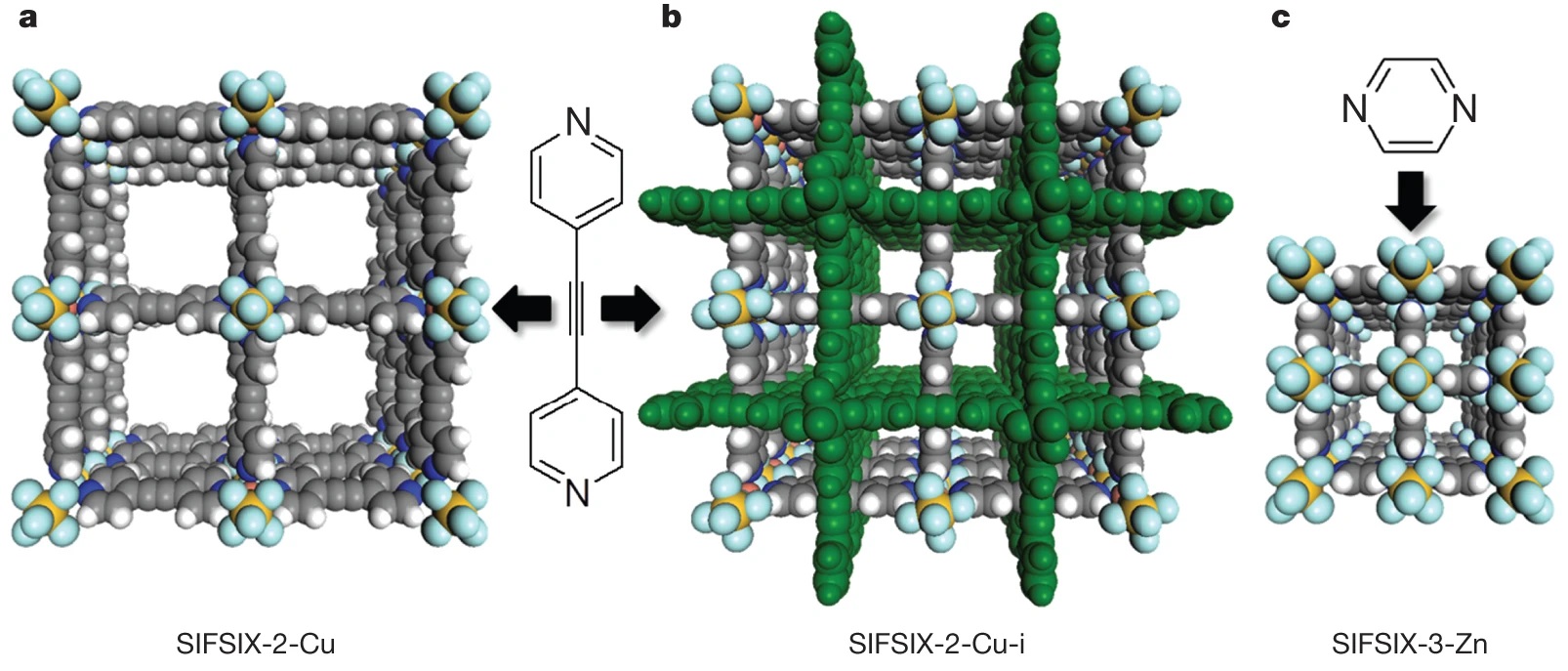
The authors from the University of South Florida (USA) and King Abdullah University of Science and Technology (KAUST, Saudi Arabia) developed three SIFSIX-series metal-organic materials (MOMs):SIFSIX-2-Cu ([Cu(dpa)₂(SiF₆)]ₙ),SIFSIX-2-Cu-i ([Cu(dpa)₂(SiF₆)]ₙ·2.5CH₃OH), andSIFSIX-3-Zn ([Zn(pyr)₂(SiF₆)]ₙ), with tunable pore sizes and high CO₂ adsorption selectivity. These materials achieved exceptional performance in industrial CO₂ separation (post-combustion, pre-combustion, natural gas upgrading) even in humid environments.
Background:
1. To address the high energy cost of CO₂ separation and limitations of existing sorbents (zeolites, amine-functionalized materials), previous researchers developed MOMs with unsaturated metal centers (UMCs) or amines for CO₂ capture. However, these materials have high regeneration energy, moisture interference (for UMCs), and decreasing selectivity with sorbate loading.
2. The authors proposed a crystal engineering/reticular chemistry strategy: designing MOMs with coordinately saturated metal centers and periodic SiF₆²⁻ (SIFSIX) pillars to precisely control pore size and functionality. This avoids UMCs/amines while achieving a "sweet spot" of CO₂ adsorption thermodynamics (moderate Qₛₜ) and kinetics (fast uptake, high selectivity).
Research Content:
1. Synthesis
The authors synthesized the three SIFSIX-series MOMs via room-temperature diffusion or direct mixing, with precursor preparation as follows:
-Precursor 1,2-bis(4-pyridyl)acetylene (dpa):
1. Add Br₂ (3.5 mL, 68 mmol) dropwise to trans-1,2-bis(4-pyridyl)ethylene (3.52 g, 19.3 mmol) in 48% HBr (46.5 mL) at 0°C, stir at 120°C for 2 h, cool to room temperature to get orange precipitate.
2. Chill in ice for 30 min, filter, wash with water, stir in 2 M NaOH (120 mL) for 30 min, filter, wash with 250 mL water, dry under vacuum to get 1,2-dibromo-1,2-bis(4-pyridyl)ethane (5.1 g, 77% yield).
3. Stir Na (2.2 g, 96 mmol) in dried t-BuOH (120 mL) at 80°C under N₂ until dissolved (20 h), add 1,2-dibromo-1,2-bis(4-pyridyl)ethane (4.0 g, 11.7 mmol) in portions, stir at 80°C for 4 h.
4. Cool to room temperature, add EtOH (20 mL) and water (20 mL), extract with CHCl₃ (4×70 mL), evaporate CHCl₃, recrystallize from toluene (overall yield 43%).
-SIFSIX-2-Cu ([Cu(dpa)₂(SiF₆)]ₙ): Room-temperature diffusion of ethanol solution of dpa (2 mL, 0.115 mmol) into ethylene glycol solution of CuSiF₆ (2 mL, 0.149 mmol) for 2 weeks, get purple rod-shaped crystals (87.4% yield based on dpa).
-SIFSIX-2-Cu-i ([Cu(dpa)₂(SiF₆)]ₙ·2.5CH₃OH):
- Method 1: Room-temperature diffusion of methanol solution of CuSiF₆ (2 mL, 0.149 mmol) into DMSO solution of dpa (2 mL, 0.115 mmol) for 1 week, get blue plate single crystals (99.8% yield based on dpa).
- Method 2: Stir methanol solution of dpa (4 mL, 0.270 mmol) with aqueous solution of CuSiF₆ (4 mL, 0.258 mmol) to get purple precipitate, heat at 85°C for 12 h (83.3% yield based on dpa).
-SIFSIX-3-Zn ([Zn(pyr)₂(SiF₆)]ₙ): Room-temperature diffusion of methanol solution of pyrazine (pyr, 2 mL, 1.3 mmol) into methanol solution of ZnSiF₆ (2 mL, 0.6 mmol) for 3 days (following known procedure) to get crystals.
2. Characterizations
1.BET and Pore Size:
- SIFSIX-2-Cu: BET surface area = 3140 m²/g (Langmuir: 3370 m²/g, N₂ adsorption at 77 K), pore size = 13.05 Å, micropore volume (calc. by Platon) = 1.10 cm³/g, experimental (t-plot) = 1.15 cm³/g.
- SIFSIX-2-Cu-i: BET surface area = 735 m²/g (Langmuir: 821 m²/g, N₂ adsorption at 77 K), pore size = 5.15 Å, micropore volume (calc.) = 0.25 cm³/g, experimental = 0.26 cm³/g.
- SIFSIX-3-Zn: BET surface area = 250 m²/g (CO₂ adsorption at 298 K, minimal N₂ uptake at 77 K), pore size = 3.84 Å.
2.XRD and Crystal Structure:
-Powder XRD (PXRD): Recorded at room temperature on Bruker D8 ADVANCE (Cu Kα, λ=1.54056 Å, 1 s/step, 0.02° step size in 2θ). Patterns match calculated ones, confirming bulk purity (Supplementary Figs 1–3).
-Single-Crystal XRD:
- SIFSIX-2-Cu: Bruker-AXS SMART-APEXII CCD (Cu Kα, λ=1.54178 Å, 100 K), tetragonal, space group P4/mmm, unit cell a=13.6316(14) Å, c=7.9680(10) Å, volume=1480.6(3) ų, Z=1, density=0.635 g/cm³ (Table S1).
- SIFSIX-2-Cu-i: Synchrotron radiation (λ=0.49594 Å, 100 K), tetragonal, space group I4/mmm, unit cell a=13.6490(11) Å, c=8.0920(6) Å, volume=1507.5(2) ų, Z=2, density=1.423 g/cm³ (Table S2).
3.Other Tests:
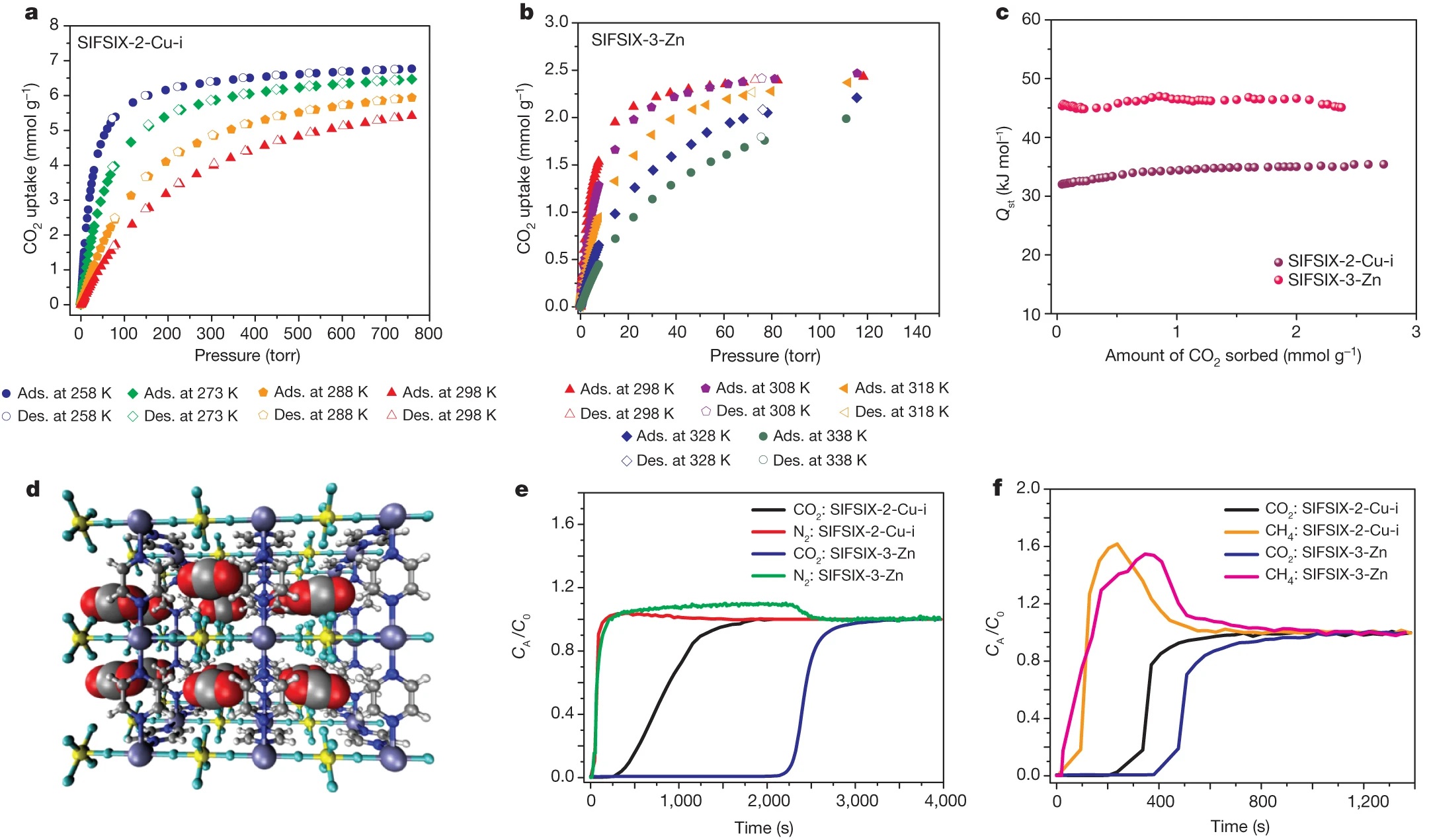
-Isosteric Heat of Adsorption (Qₛₜ): Calculated via Clausius-Clapeyron (SIFSIX-2-Cu: 22 kJ/mol; SIFSIX-2-Cu-i: 31.9 kJ/mol; SIFSIX-3-Zn: 45 kJ/mol) and virial equation, consistent with pore size effects.
-Thermal Stability: VT-PXRD (173 K–573 K under vacuum) shows SIFSIX-2-Cu-i retains crystallinity up to 573 K, SIFSIX-3-Zn up to 523 K.
-Moisture Stability: SIFSIX-2-Cu-i PXRD unchanged at 5–95% RH; SIFSIX-3-Zn has reversible phase change at >35% RH (regenerated by heating 323–373 K under vacuum).
-Water Adsorption: Type I isotherms, 20 wt% (SIFSIX-2-Cu-i) and 11 wt% (SIFSIX-3-Zn) uptake at 74% RH (298 K).
3. Application
The MOMs were tested for CO₂ separation in industrial scenarios, with results:
1.Post-combustion Capture (CO₂/N₂:10/90, 298 K, 1 bar):
- SIFSIX-2-Cu-i: CO₂ uptake = 70 mg/g (breakthrough), selectivity = 72 (breakthrough) / 140 (IAST); 74% RH reduces uptake to 55 mg/g, selectivity to 191 (vs. 237 dry).
- SIFSIX-3-Zn: CO₂ uptake = 99.9–104.4 mg/g, selectivity = 495 (breakthrough) / 1818 (IAST); N₂ breakthrough in seconds, CO₂ retention ~2000 s (vs. 300 s for SIFSIX-2-Cu-i).
2.Pre-combustion Capture (CO₂/H₂:30/70, 298 K, 1 bar):
- SIFSIX-2-Cu-i: CO₂ uptake = 85 mg/g, selectivity = 240 (breakthrough); 74% RH reduces uptake to 1.61 mmol/g (vs. 1.99 mmol/g dry).
- SIFSIX-3-Zn: CO₂ uptake similar to pure CO₂, selectivity >1800 (gravimetric), suitable for H₂ purification.
3.Natural Gas Upgrading (CO₂/CH₄:50/50 or 5/95, 298 K, 1 bar):
- SIFSIX-2-Cu-i: CO₂ uptake = 183 mg/g (breakthrough), selectivity = 51 (breakthrough) / 33 (IAST) (50/50 mixture).
- SIFSIX-3-Zn: CO₂ uptake = 108–110 mg/g, selectivity = 109 (breakthrough) / 231 (IAST) (50/50 mixture); outperforms Mg-dobdc and zeolite 13X.
4.Cyclability: SIFSIX-3-Zn maintains CO₂ uptake/selectivity over multiple vacuum swing regeneration cycles (323 K, 0.15 bar).
4. Mechanism
-Thermodynamic Driving Force: Periodic SiF₆²⁻ pillars provide negatively charged F atoms, which electrostatically interact with electropositive C atoms of CO₂ (GCMC simulations, Supplementary Fig. 26). Narrower pores (SIFSIX-3-Zn > SIFSIX-2-Cu-i > SIFSIX-2-Cu) enhance attractive potential field overlap, increasing Qₛₜ and CO₂ affinity.
-Kinetic Selectivity: CO₂ (kinetic diameter ~3.3 Å) fits into ultra-micropores of SIFSIX-3-Zn (3.84 Å) and SIFSIX-2-Cu-i (5.15 Å), while larger gases (N₂: 3.64 Å, CH₄: 3.8 Å) are excluded/weakly adsorbed. Kinetic studies show CO₂ uptake faster than N₂, O₂, CH₄, H₂ (Supplementary Fig. 11).
-Moisture Tolerance: Water adsorption is reduced in CO₂-containing mixtures; SIFSIX-3-Zn’s reversible phase change avoids structural collapse, SIFSIX-2-Cu-i is structurally unchanged by moisture (PXRD, Supplementary Fig. 17).
Outlook:
This research shows crystal engineering of SIFSIX-series MOMs—via pore size tuning and SiF₆²⁻-mediated electrostatic interactions—balances CO₂ adsorption thermodynamics (moderate Qₛₜ for low regeneration energy) and kinetics (high selectivity, fast uptake). The materials outperform traditional sorbents (zeolites, amine-MOFs) in industrial CO₂ separation, with unprecedented selectivity (e.g., SIFSIX-3-Zn: CO₂/N₂ selectivity >1800) and moisture stability. This strategy provides a modular approach to design porous materials for gas separation, addressing global CO₂ emission and industrial gas purification needs.
Porous materials with optimal adsorption thermodynamics and kinetics for CO₂ separation
Authors: Patrick Nugent, Youssef Belmabkhout, Stephen D. Burd, Amy J. Cairns, Ryan Luebke, Katherine Forrest, Tony Pham, Shengqian Ma, Brian Space, Lukasz Wojtas, Mohamed Eddaoudi, Michael J. Zaworotko
DOI: 10.1038/nature11893
Link: https://www.nature.com/articles/nature11893
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

