Home >
News > A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate-Metal Organic Framework for Aerobic Decontamination
A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate-Metal Organic Framework for Aerobic Decontamination
Summary:
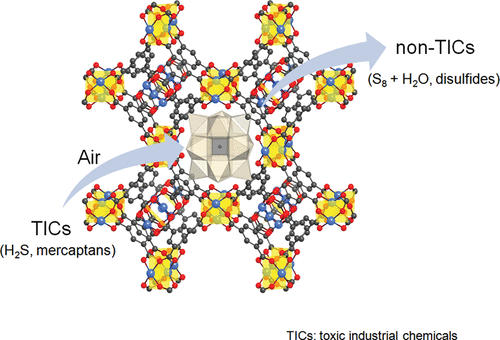
The authors from Emory University (USA) and the University of California, Los Angeles (USA) developed a polyoxometalate-metal organic framework (POM-MOF) material[Cu₃(C₉H₃O₆)₂]₄[(CH₃)₄N]₄CuPW₁₁O₃₉H·40H₂O (1) with synergistic stability and reactivity, achieving efficient aerobic decontamination of toxic sulfur compounds (e.g., H₂S, thiols) in environmental pollution control.
Background:
1. To address two key challenges in MOF catalysis—developing MOF derivatives for ambient-air-driven catalysis and enhancing MOF catalyst stability—previous researchers incorporated polyoxometalates (POMs) into MOFs via covalent/electrostatic methods for oxidation catalysis. However, these materials either showed low catalytic activity, poor POM retention, or insufficient stability (e.g., MOFs decompose in narrow pH ranges, POMs hydrolyze in basic solutions).
2. The authors proposed an innovative strategy: matching the size of Keggin-type POM ([CuPW₁₁O₃₉]⁵⁻) with the pores of MOF-199 (HKUST-1) to form a POM-MOF composite. This design not only improves the stability of both components but also boosts the POM’s catalytic turnover rate for aerobic oxidations by two orders of magnitude.
Research Content:
1. Synthesis
The authors synthesized material 1 via hydrothermal method:
- Precursor mixture: Cu(NO₃)₂·2.5H₂O, K₅[CuPW₁₁O₃₉], trimesic acid, and (CH₃)₄NOH in distilled water (pH ~3).
- Reaction conditions: Teflon-lined Parr bomb, 200°C for 16 h, programmed cooling to 100°C for 4 h, then ambient cooling.
- Purification: Washing with distilled water, soaking in saturated KCl solution, sonication, and vacuum drying. Yield: ~25% (based on POM).
2. Characterizations
1.BET and Pore Size:
- BET surface area of 1: 462 m²/g (1/3 of pure MOF-199’s 1264 m²/g), indicating POM/counterions occupy most MOF pores.
- Nitrogen adsorption isotherms (77 K) confirm porosity, with interconnected nonlinear channels for reactant/product transport.
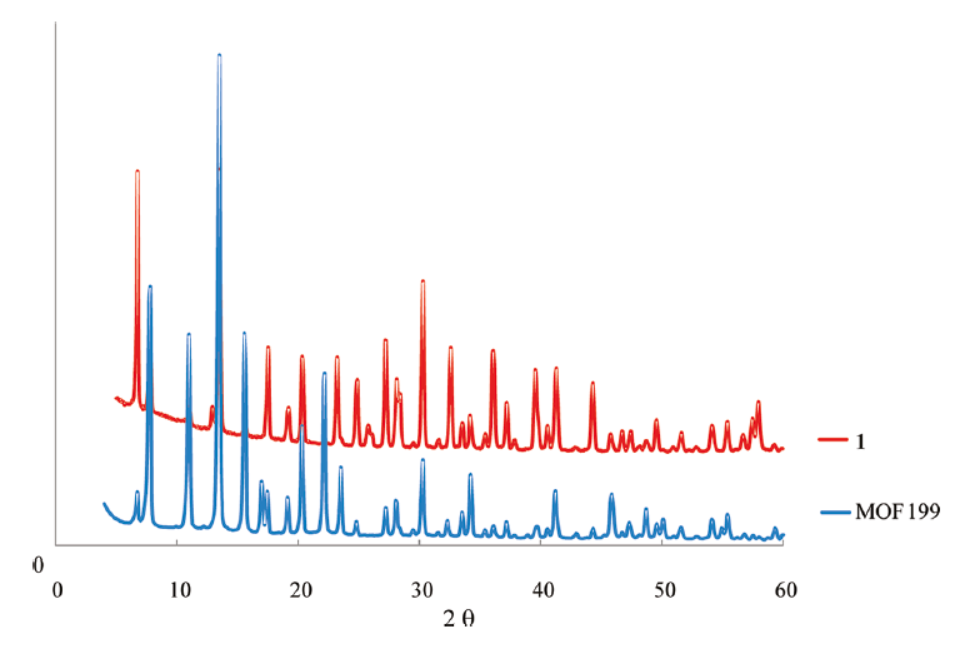
2.XRD and Crystal Structure:
- Powder XRD (Cu Kα radiation) shows 1 retains MOF-199’s framework.
- Single-crystal XRD (173 K, Mo Kα radiation): Cubic system (space group Fm$\overline{3}$m), unit cell a=26.307(2) Å, Z=4. POMs occupy 50% of large pores, (CH₃)₄N⁺ counterions in adjacent small pores; POMs are orientationally disordered.
3.Spectroscopic and Thermal Tests:
- FT-IR: Characteristic peaks of both POM (e.g., 1080, 987 cm⁻¹ for W-O/P-O) and MOF-199 (e.g., 1649, 1374 cm⁻¹ for carboxylate) confirm component integration.
- TGA: Weight loss matches calculated content of water + (CH₃)₄N⁺ (16.46%) and MOF framework (26.82%).
- UV-vis/ICP: No POM leaching from 1 (Cu content in filtrate: 0.3 mg/Kg vs. 43.9 mg/Kg for pure MOF-199).
3. Application
1.Aerobic H₂S Oxidation:
- Aqueous phase: 1 catalyzes H₂S → 1/8 S₈ + H₂O (pH 4.8, room temperature) with TON ~4000 in <20 h (air/O₂; pure POM/MOF-199 show no activity).
- Gas phase: TON=12 (limited by S₈ pore blockage); PW₁₂-MOF/MOF-199 have negligible activity.
2.Aerobic Thiol Oxidation:
- Converts thiols to disulfides (2RSH + 1/2 O₂ → RSSR + H₂O) in chlorobenzene (45°C).
- 99% chemo-selectivity; shape/size selectivity: 2-hydroxyethanethiol (95% conversion) > short-chain thiols > sterically hindered thiols (e.g., p-toluenethiol <30% conversion).
- TON of 1 is ~10× higher than TBA₅[CuPW₁₁O₃₉] (homogeneous) or POM-MOF mechanical mixture.
3.Stability:
- 1 remains intact at pH 11.0 for 12 h (FT-IR/UV-vis), while pure POM decomposes at pH >5.9 and MOF-199 decomposes in H₂S solution (pH 4.8) within minutes.
- Reusable for 3 cycles without activity loss or structural damage (XRD/FT-IR post-reaction).
4. Mechanism
-Synergistic Stability: Electrostatic interactions between MOF’s Cu(II) centers and POM’s negative charge ([CuPW₁₁O₃₉]⁵⁻) stabilize both components, expanding POM’s pH tolerance (pH 2–11) and MOF’s hydrolytic stability.
-Catalytic Enhancement: Electrostatic interactions increase the reduction potential of POM’s Cu centers, accelerating POM reduction (rate-limiting step); reoxidation of Cu(I) by O₂ is not rate-limiting (oxidation rate independent of O₂ pressure).
-Shape Selectivity: MOF pores restrict access of sterically large thiols to POM active sites, leading to size-dependent conversion.
Outlook:
This research successfully develops a robust POM-MOF catalyst with mutual stability enhancement and high aerobic catalytic activity. It provides a scalable strategy for designing composite materials by matching POM/MOF sizes, enabling efficient, solvent-free decontamination of toxic industrial chemicals (TICs) using only ambient air. The work opens avenues for tailored POM-MOF combinations to target diverse pollutants, with potential applications in environmental remediation and industrial deodorization.
A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate-Metal Organic Framework for Aerobic Decontamination
Authors: Jie Song, Zhen Luo, David K. Britt, Hiroyasu Furukawa, Omar M. Yaghi, Kenneth I. Hardcastle, Craig L. Hill
DOI: 10.1021/ja203695h
Link: https://pubs.acs.org/doi/10.1021/ja203695h
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

