Home >
News > High-Capacity Hydrogen and Nitric Oxide Adsorption and Storage in a Metal-Organic Framework
High-Capacity Hydrogen and Nitric Oxide Adsorption and Storage in a Metal-Organic Framework
Summary:
The authors from the University of St. Andrews (UK), University of Newcastle upon Tyne (UK), UHI Millennium Institute (UK), University of Edinburgh (UK), and University of Torino (Italy) developed a metal-organic framework (MOF) material HKUST-1 with high porosity and open copper sites, achieving excellent performance in hydrogen (H₂) and nitric oxide (NO) adsorption/storage, especially showing potential in energy storage and biological antithrombotic applications.
Background:
1. To address the challenges of low gas adsorption capacity, poor stability, and limited applicability of existing porous materials (such as metal hydrides, carbon nanotubes, zeolites, and organic polymers) in H₂ storage (for zero-emission energy) and NO storage (for environmental and biological applications), previous researchers have conducted extensive studies. For example, some MOFs showed certain H₂ adsorption capacity, and zeolites could adsorb NO, yet these materials had shortcomings: H₂ adsorption of most MOFs was below commercial application requirements, and NO adsorption capacity of zeolites was only around 1 mmol·g⁻¹ at 298 K, which was insufficient for high-demand scenarios.
2. The authors in this study proposed to use HKUST-1 (a copper benzene tricarboxylate MOF) with open copper sites and high porosity, and through systematic synthesis, characterization, and application tests, obtained high H₂ and NO adsorption capacities, and verified its biological activity in inhibiting platelet aggregation.
Research Content:
1. Synthesis
The authors synthesized HKUST-1 using a solvothermal method: 3.0 mmol of Cu(NO₃)₂·3H₂O (0.716 g) and 2.0 mmol of benzene 1,3,5-tricarboxylic acid (0.421 g) were mixed with 12 mL of EtOH/H₂O (50:50) solution in a Teflon-lined autoclave. The mixture was stirred at ambient temperature for 30 minutes, then heated in a preheated oven at 383 K for 24 hours. After crystallization and natural cooling, the product was sonicated in EtOH/H₂O (50:50) for 5-10 minutes, isolated by Buchner filtration, and dried in air for 1 day to obtain large turquoise blue crystals.
2. Characterizations
1)BET and pore size distribution: N₂ adsorption (77 K) and CO₂ adsorption (195 K) gave pore volumes of 0.684 cm³·g⁻¹ and 0.703 cm³·g⁻¹, respectively, which were consistent with the crystallographic pore volume (~0.72 cm³·g⁻¹) calculated by PLATON, confirming high porosity.
2)SEM/TEM tests: Not mentioned in the article.
3)Other tests:
-Powder X-ray diffraction (PXRD): Collected on a STOE STADIP diffractometer (Cu Kα₁ radiation, λ=1.54060 Å); the pattern of activated HKUST-1 matched the theoretical pattern from single crystal data, confirming high crystallinity and no impurities.
-FTIR: Measured on a PerkinElmer Spectrum GX FT-IR system (resolution 4.0 cm⁻¹, KBr disc, ~3 wt% sample); characteristic peaks included 3443.26 cm⁻¹ (O-H stretch), 1703.49 cm⁻¹ (carboxylate asymmetric stretch), etc.; after NO adsorption, a new peak at 1887 cm⁻¹ appeared, indicating Cu(II)···NO adduct formation.
-Thermogravimetric analysis (TGA): Conducted on a TA Instruments SDT 2960 analyzer (10 K·min⁻¹ heating rate, up to 1073 K, argon atmosphere); weight losses occurred at ~27.94°C (moisture loss), ~125.96°C, and ~249.67°C (guest molecule and framework decomposition).
-Gravimetric adsorption system: Used for H₂/D₂/NO adsorption; the microbalance had a sensitivity of 0.1 μg and reproducibility of 0.01% of the load, ensuring accurate adsorption capacity measurement.
3. Application
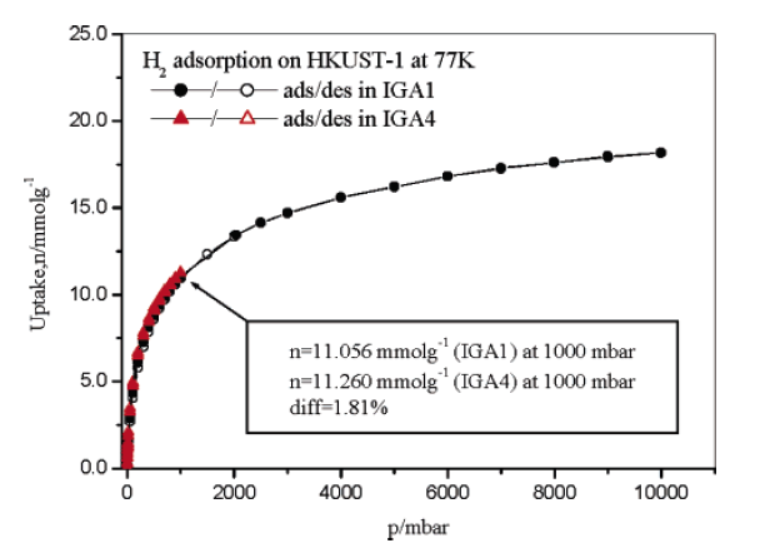
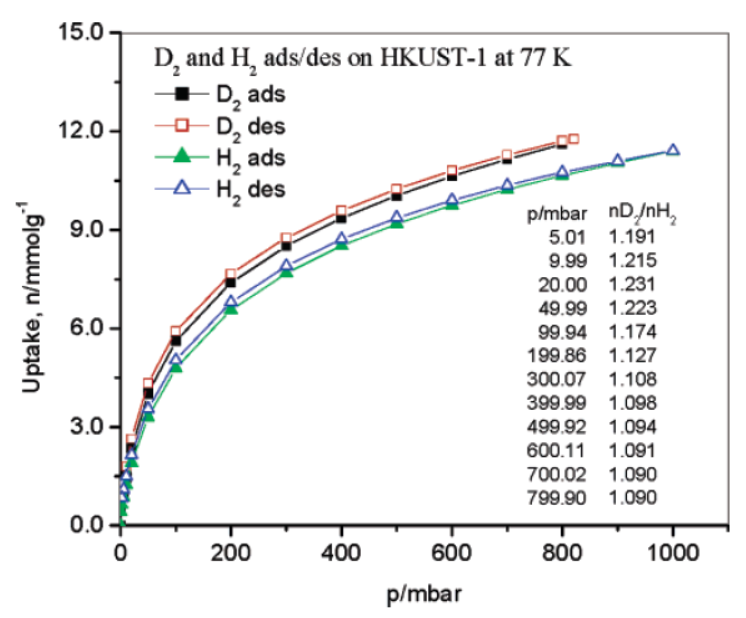
1)H₂ storage application: At 77 K, H₂ adsorption capacity was ~11.16 mmol·g⁻¹ (2.27 wt%) at 1 bar and ~18 mmol·g⁻¹ (3.6 wt%) at 10 bar; D₂ adsorption was 1.09-1.20 times that of H₂ (depending on pressure), and the isotherm repeatability was within 1.8%, showing potential for low-temperature H₂ storage.
2)NO storage and biological application: At 196 K (1 bar), NO adsorption capacity was ~9 mmol·g⁻¹ (higher than any reported porous solid); at 298 K (1 bar), it was just over 3 mmol·g⁻¹. Chemiluminescence tests showed NO release on contact with water vapor; platelet aggregometry tests confirmed that NO-loaded HKUST-1/PTFE disks completely inhibited platelet aggregation in platelet-rich plasma (PRP), with the inhibitory effect eliminated by NO-scavenger oxyhemoglobin.
4. Mechanism
1)H₂ adsorption mechanism: H₂ is weakly adsorbed in HKUST-1 pores through van der Waals forces, so significant adsorption only occurs at low temperature (77 K); the density of adsorbed H₂ (~0.057 g·cm⁻³) is lower than liquid H₂ (0.0708 g·cm⁻³ at 20.2 K), and D₂ adsorption is higher than H₂ due to quantum mechanical effects.
2)NO adsorption and release mechanism: NO is both physisorbed in pores and chemisorbed on open copper sites of HKUST-1 to form Cu(II)···NO adducts (proven by FTIR peak at 1887 cm⁻¹), leading to isotherm hysteresis; ~2.21 mmol·g⁻¹ NO is irreversibly adsorbed (1 NO per dicopper(II) tetracarboxylate unit). On contact with water (e.g., in PRP), NO is released from the adduct, and the released amount, though lower than initial adsorption, is sufficient for biological activity.
Outlook:
This research systematically characterizes HKUST-1's structure and verifies its ultrahigh H₂ and NO adsorption capacities, confirming MOFs' potential in energy storage (H₂), environmental protection (NOx traps), and biological antithrombosis. However, HKUST-1's poor stability in physiological solutions (copper dissolution) and unstudied toxicology need further improvement. This work provides a basis for the design and application of high-performance MOF-based gas storage materials.
High-Capacity Hydrogen and Nitric Oxide Adsorption and Storage in a Metal-Organic Framework
Authors: Bo Xiao, Paul S. Wheatley, Xuebo Zhao, Ashleigh J. Fletcher, Sarah Fox, Adriano G. Rossi, Ian L. Megson, S. Bordiga, L. Regli, K. Mark Thomas, Russell E. Morris
DOI: 10.1021/ja066098k
Link: https://pubs.acs.org/doi/10.1021/ja066098k
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

