Home >
News > A Series of Highly Stable Mesoporous Metalloporphyrin Fe-MOFs
A Series of Highly Stable Mesoporous Metalloporphyrin Fe-MOFs
Summary:
The authors from Texas A&M University and Stockholm University developed a series of mesoporous metalloporphyrin Fe-MOFs (PCN-600(M), M=Mn, Fe, Co, Ni, Cu) with large 1D channels (3.1 nm), high pore volume (up to 1.80 cm³ g⁻¹), and excellent chemical stability (pH 2-11), achieving effective peroxidase-mimetic catalysis in co-oxidation reactions.
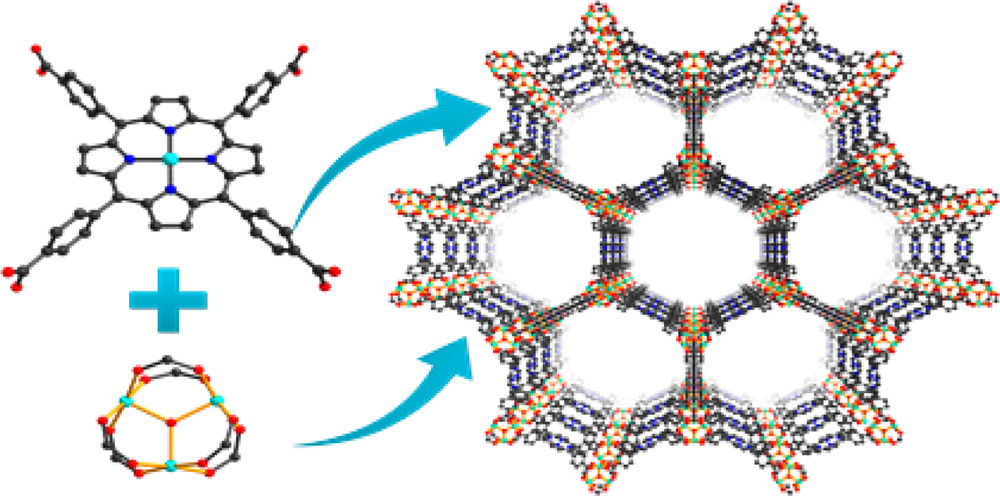
Background:
1. To address the poor chemical stability of porphyrinic MOFs with soft Lewis acidic nodes and the difficulty in phase purification of porphyrinic Zr-MOFs, previous researchers explored MOFs with harder Lewis acidic clusters but faced challenges in synthesis and structure determination.
2 The authors proposed using the preassembled [Fe₃O(OOCCH₃)₆] building block and tetrakis(4-carboxyphenyl)porphyrin (TCPP) linker to synthesize PCN-600 series, obtaining highly stable mesoporous porphyrinic Fe-MOFs with phase purity.
Research Content:
1. Synthesis:
The authors synthesized PCN-600(M) via solvothermal reactions of M-TCPP (M=Mn, Fe, Co, Ni, Cu), [Fe₃O(OOCCH₃)₆(OH)]·2H₂O, and trifluoroacetic acid in DMF at 150°C for 12 h.
2. Characterizations:
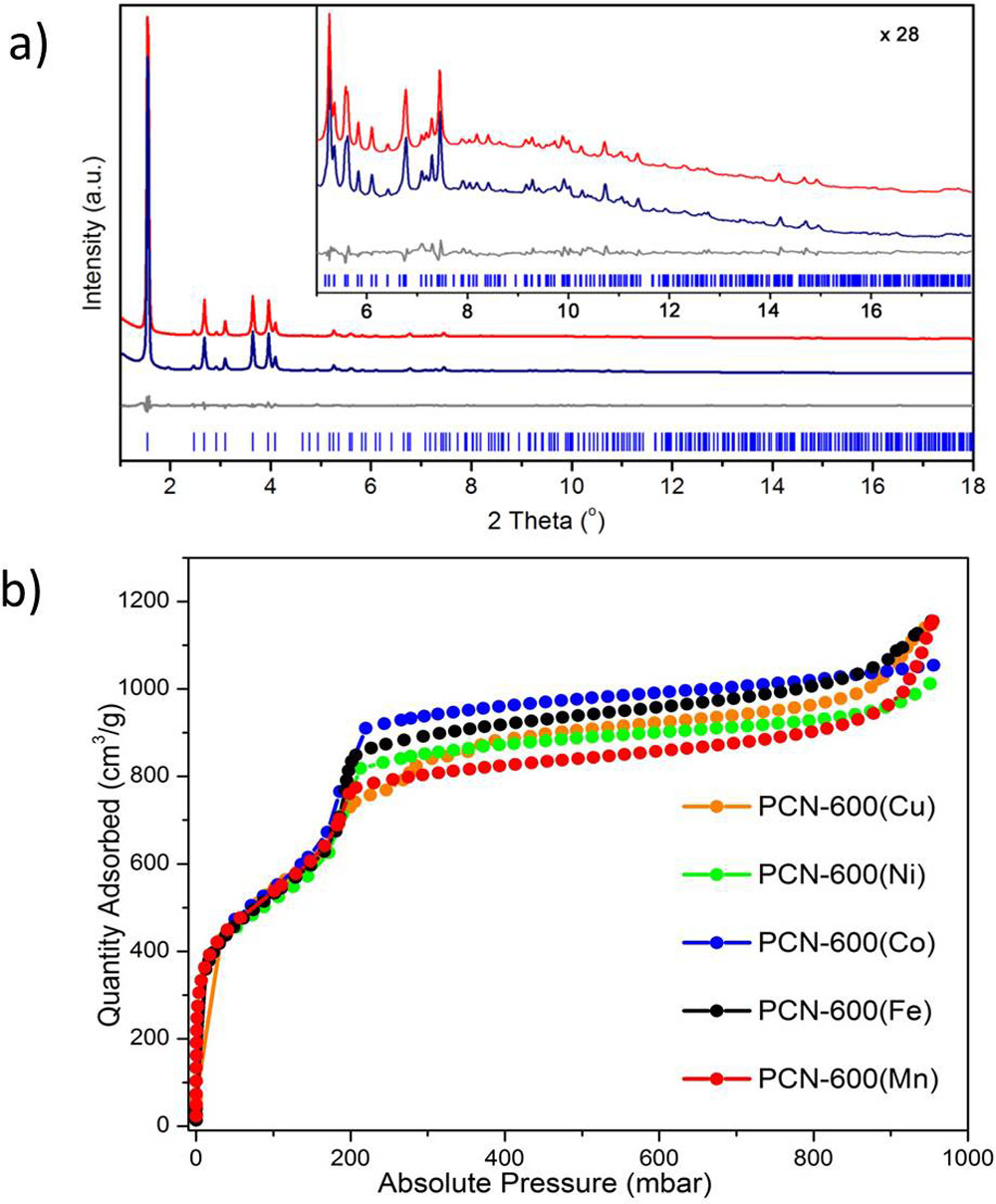
1) BET results show PCN-600 series have surface areas up to 2350 m² g⁻¹, with PCN-600(Fe) exhibiting a total pore volume of 1.80 cm³ g⁻¹; DFT simulation indicates a single type of 2.6 nm pores.
2) No SEM/TEM particle size data provided; needle-shaped single crystals (length ~0.3 mm) were obtained.
3) PXRD confirms the stp-a topology with space group P6/mmm and unit cell parameters a=b=31.27 Å, c=16.95 Å; TGA shows decomposition at ~320°C; UV-vis spectra characterize catalytic product formation.
3. Application:
PCN-600(Fe) was tested as a peroxidase mimic for co-oxidation of phenol and 4-AAP with H₂O₂, showing a Michaelis-Menten constant (Kₘ) of 6.37 mM, Vₘₐₓ of 0.042 mM·min⁻¹, and catalytic efficiency (k꜀ₐₜ/Kₘ) of 0.10 min⁻¹·mM⁻¹, comparable to cytochrome c.
4. Mechanism:
The high chemical stability arises from strong coordination bonds between Fe³⁺ (hard Lewis acid) and carboxylate groups in the [Fe₃O(OOC)₆] cluster. The mesoporous channels (3.1 nm) expose porphyrinic active sites, enhancing substrate affinity and enabling efficient catalysis.
Outlook:
This research provides a stable and porous platform for enzyme-mimetic catalysis, especially under harsh conditions, and paves the way for applications in sensing, biomimetic catalysis, and other fields requiring stable mesoporous materials.
A Series of Highly Stable Mesoporous Metalloporphyrin Fe-MOFs
Authors: Kecheng Wang, Dawei Feng, Tian-Fu Liu, Jie Su, Shuai Yuan, Ying-Pin Chen, Mathieu Bosch, Xiaodong Zou, Hong-Cai Zhou
DOI: 10.1021/ja507269n
Link: https://pubs.acs.org/doi/10.1021/ja507269n
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

