Home >
News > Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway
Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway
Summary:
The authors from Southwest Petroleum University, Wuhan University of Technology, and Guangzhou University developed MIL-88A, a Fe-based MOF with visible light response, porous structure, and good stability, achieving efficient degradation of tetracycline hydrochloride (TC-HCl) in the field of environmental pollutant treatment.
Background:
1. Antibiotic pollution, especially tetracycline, is a serious problem due to its stable structure and resistance to biological degradation. Previous methods like adsorption, biological processes, and single advanced oxidation processes (AOPs) or photocatalysis have limitations such as low efficiency and rapid electron-hole recombination.
2. The authors proposed coupling heterogeneous AOPs and photocatalysis using MIL-88A as a co-catalyst, which significantly enhanced the degradation efficiency of high-concentration TC.
Research Content:
1. Synthesis:
The authors synthesized MIL-88A using a hydrothermal method with FeCl₃·6H₂O and fumaric acid as precursors, reacted in ultrapure water at 65 ℃ for 12 h.
2. Characterizations:
1) BET surface area of MIL-88A is 15.95 m²/g, total pore volume is 0.11 cm³/g, with a combination of micropores, mesopores, and a small number of macropores.
2) SEM tests show MIL-88A has well-defined hexagonal microrods morphology, with length over 1 μm and diameter <400 nm.
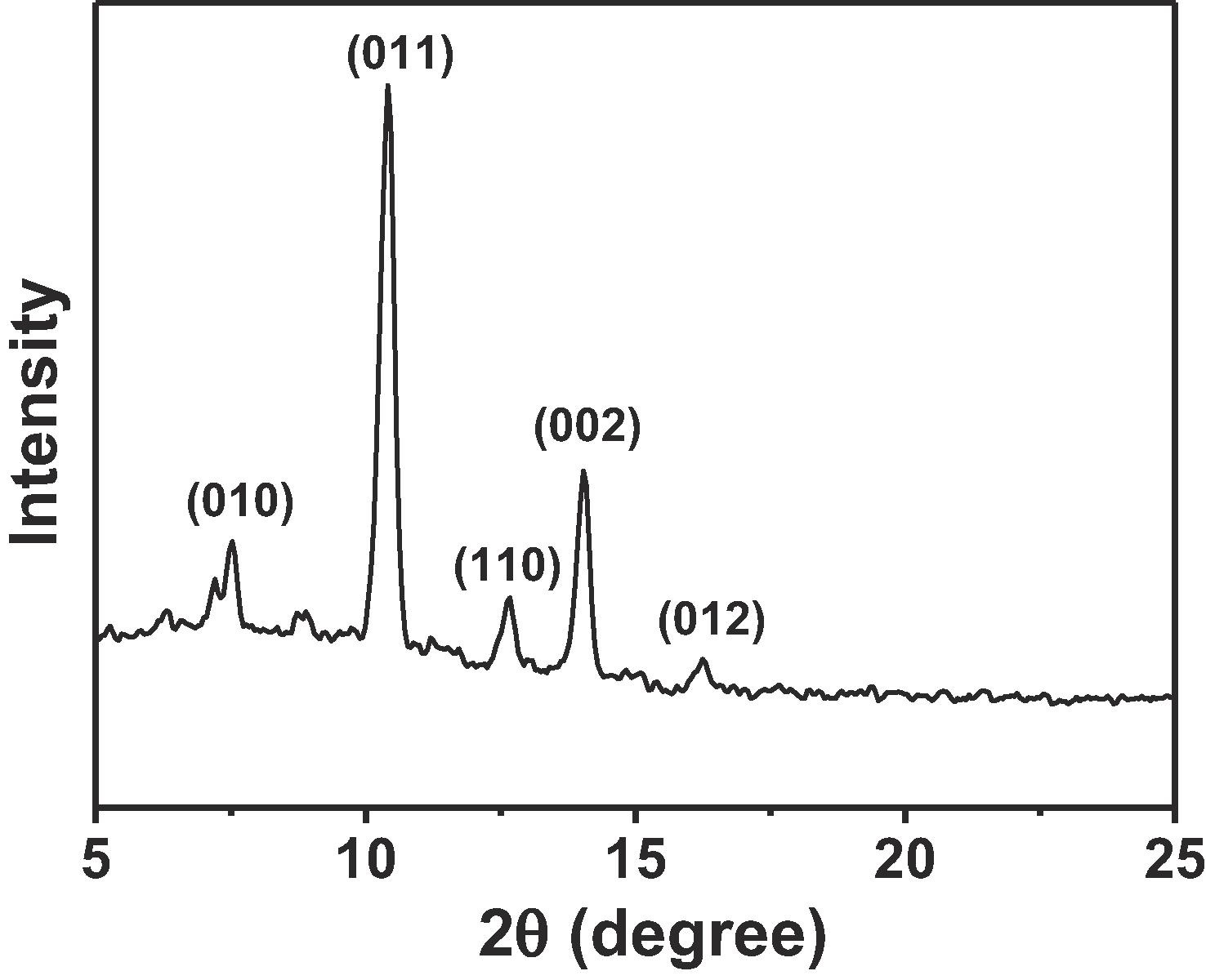
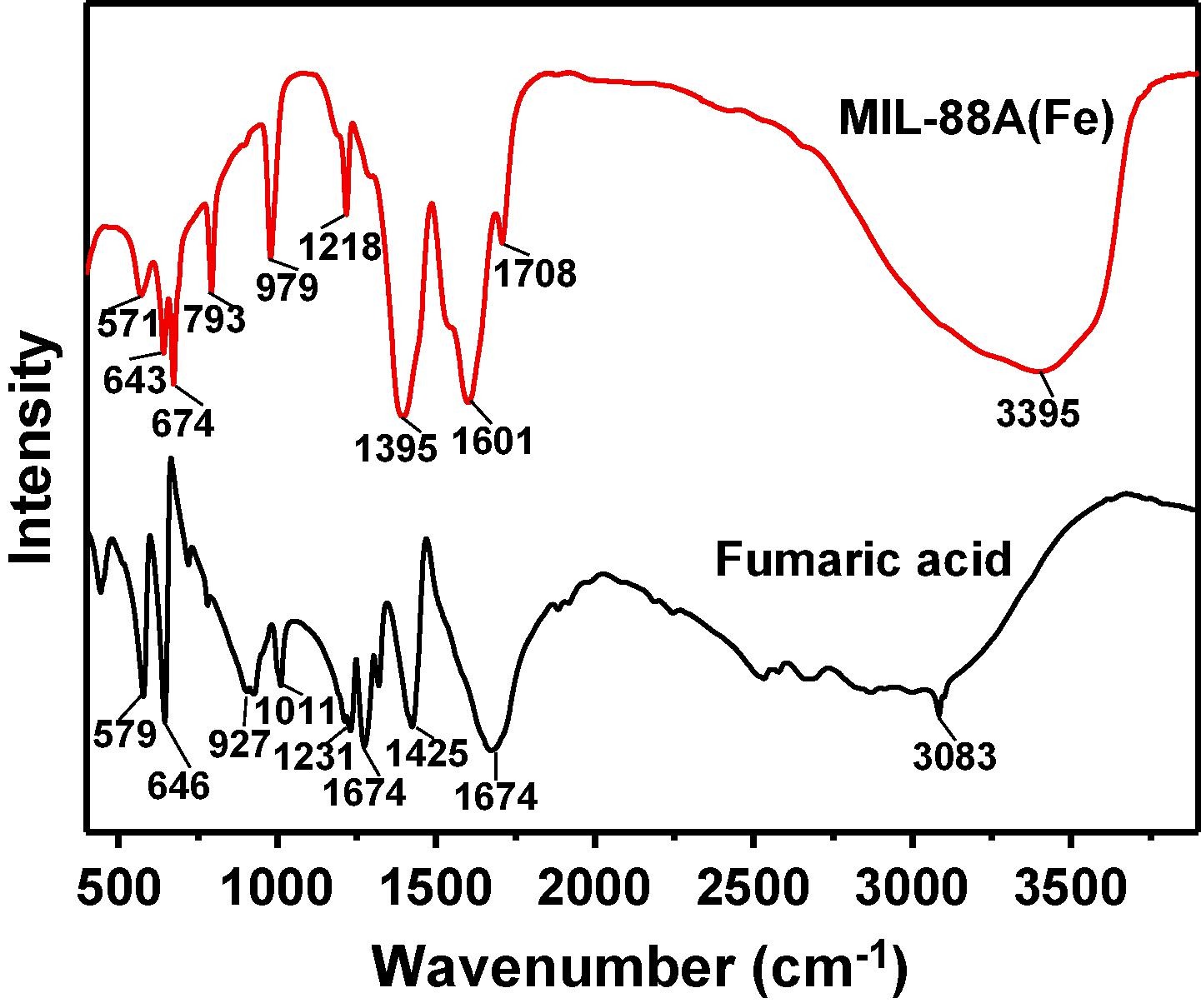
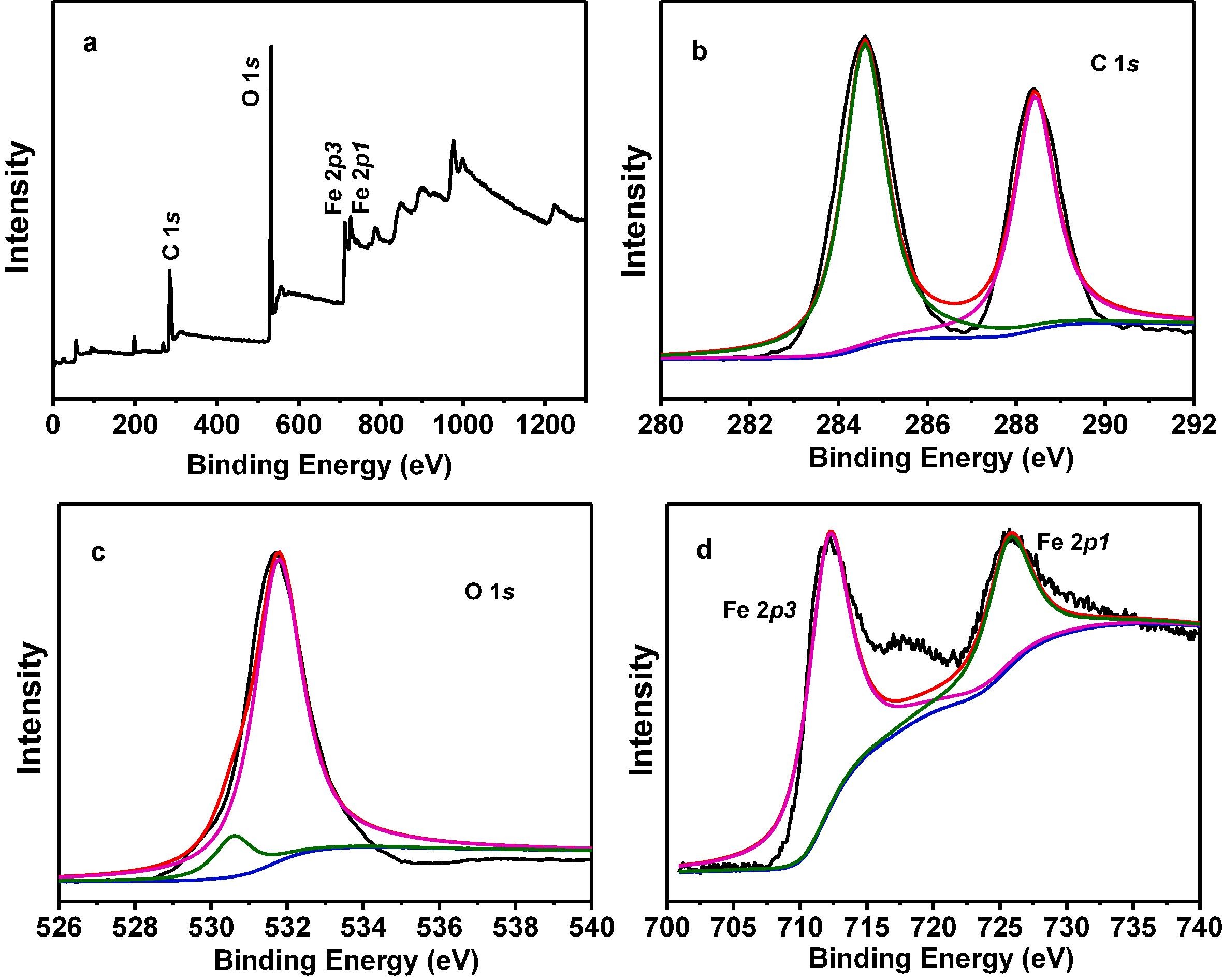
3) Other tests: XRD confirmed its successful synthesis; FTIR indicated the presence of carboxyl groups and Fe-O bonds; XPS showed it contains Fe, C, O elements with Fe in +3 state; UV-vis DRS revealed it can absorb visible light with a band gap of 2.25 eV; Mott-Schottky plot indicated it is an n-type semiconductor with conduction band potential of -0.41 V vs. NHE and valence band potential of 1.84 V vs. NHE.
3. Application:
The material was tested in TC-HCl degradation. Under optimal conditions (0.25 g/L MIL-88A, 4 mM PS, initial pH=3.45, visible light), 200 mg/L TC was completely degraded within 80 min, with a pseudo-first-order kinetic constant of 0.0396 min⁻¹. It also showed good stability after 5 cycles.
4. Mechanism:
PS acts as an electron acceptor to suppress electron-hole recombination. Photogenerated electrons from MIL-88A under visible light activate PS to generate SO₄⁻• radicals. Fe(III) in MIL-88A can also activate PS to produce SO₄⁻• radicals. SO₄⁻• and •O₂⁻ are the predominant radicals for TC degradation. LC-MS identified 18 intermediates, and the degradation pathway involves N-demethylation, oxidation of double bonds, and hydrolysis, etc.
Outlook:
This research successfully coupled heterogeneous AOPs and photocatalysis using MIL-88A, providing a new efficient method for the degradation of organic pollutants like tetracycline, which is of great significance for environmental purification.
Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: synergistic effect and degradation pathway
Authors: Ying Zhang, Jiabin Zhou, Xin Chen, Luo Wang, Weiquan Cai
DOI: 10.1016/j.cej.2019.03.108
Link: https://www.sciencedirect.com/science/article/pii/S1385894719305741
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.


