Home >
News > Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent
Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent
Summary:
The authors from a b College of Environmental Science and Engineering, Hunan University, Changsha 410082, China; c Department of Civil and Environmental Engineering, University of Connecticut, Storrs, CT 06269, USA developed multi-walled carbon nanotube functionalized MIL-53(Fe) (MWCNT/MIL-53(Fe)) nanocomposite with characteristics of large specific surface area, good thermal stability, and excellent water stability, achieving efficient adsorption of tetracycline antibiotics (TCN, OTC, CTC) from aqueous solutions.
Background:
1. Tetracycline antibiotics (TCS) are persistent pollutants in water, and adsorption is an effective removal method, but existing adsorbents have limited capacity. Metal-organic frameworks (MOFs) have potential but poor water stability, restricting their application.
2. The authors synthesized MWCNT/MIL-53(Fe) by combining MWCNT with MIL-53(Fe), which improved stability and adsorption performance, showing high adsorption capacity for TCS.
Research Content:
1. Synthesis:
The authors synthesized MIL-53(Fe) via solvothermal method by reacting FeCl₃·6H₂O and 1,4-BDC in DMF at 170°C for 24 h. MWCNT was purified with mixed acid, then mixed with MIL-53(Fe) precursors in different proportions (1-30 wt%) and reacted under the same conditions to obtain MWCNT/MIL-53(Fe), with 20% MWCNT as optimal.
2. Characterizations:
1) BET and pore size distribution: MWCNT/MIL-53(Fe) has a BET surface area of 60.17 m²/g, total pore volume of 0.182 cm³/g, and average pore size of 12.09 nm, larger than MIL-53(Fe).
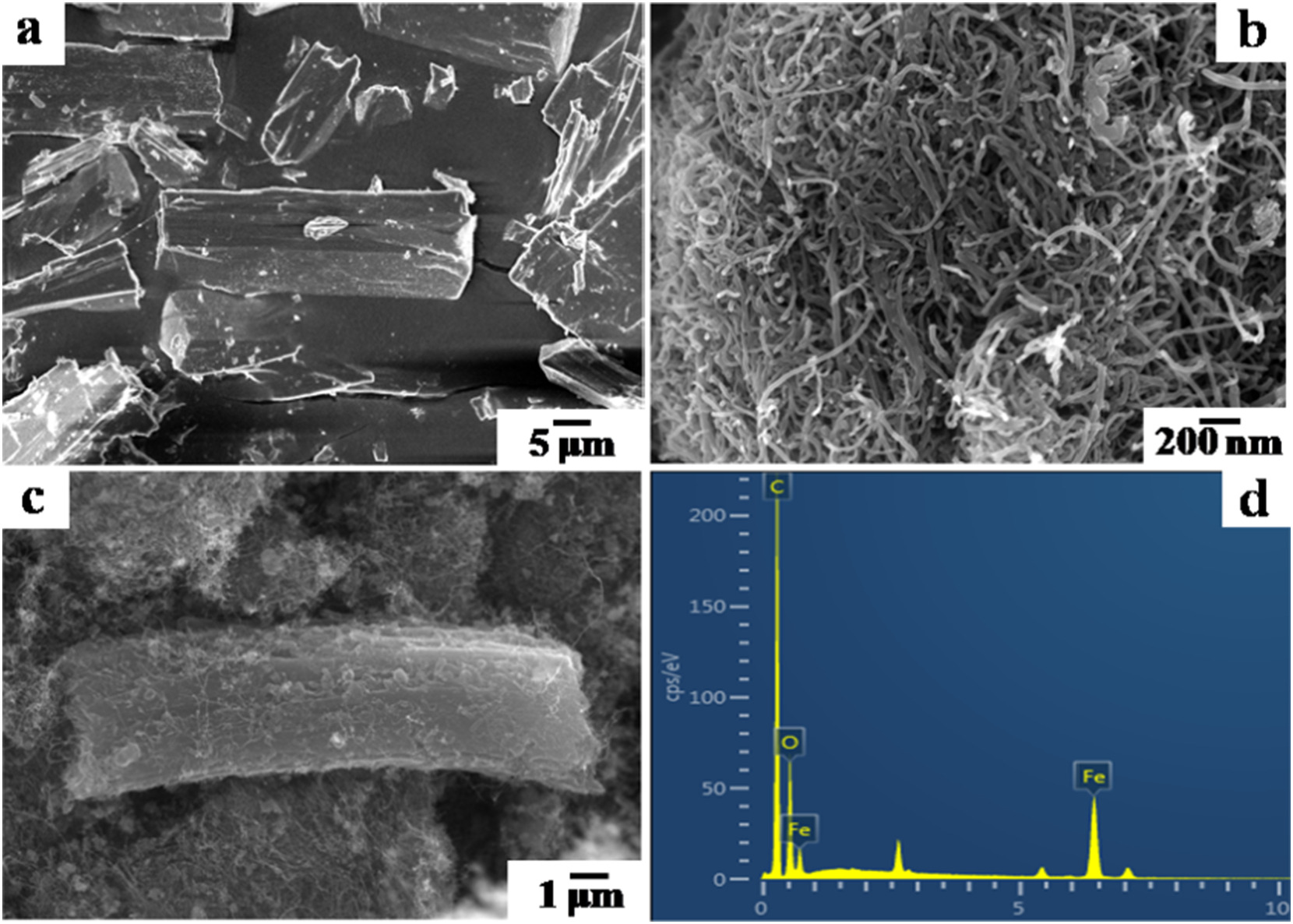
2) SEM tests: MIL-53(Fe) is rod-shaped; MWCNT is tubular; MWCNT/MIL-53(Fe) shows intimate contact between MWCNT and MIL-53(Fe).
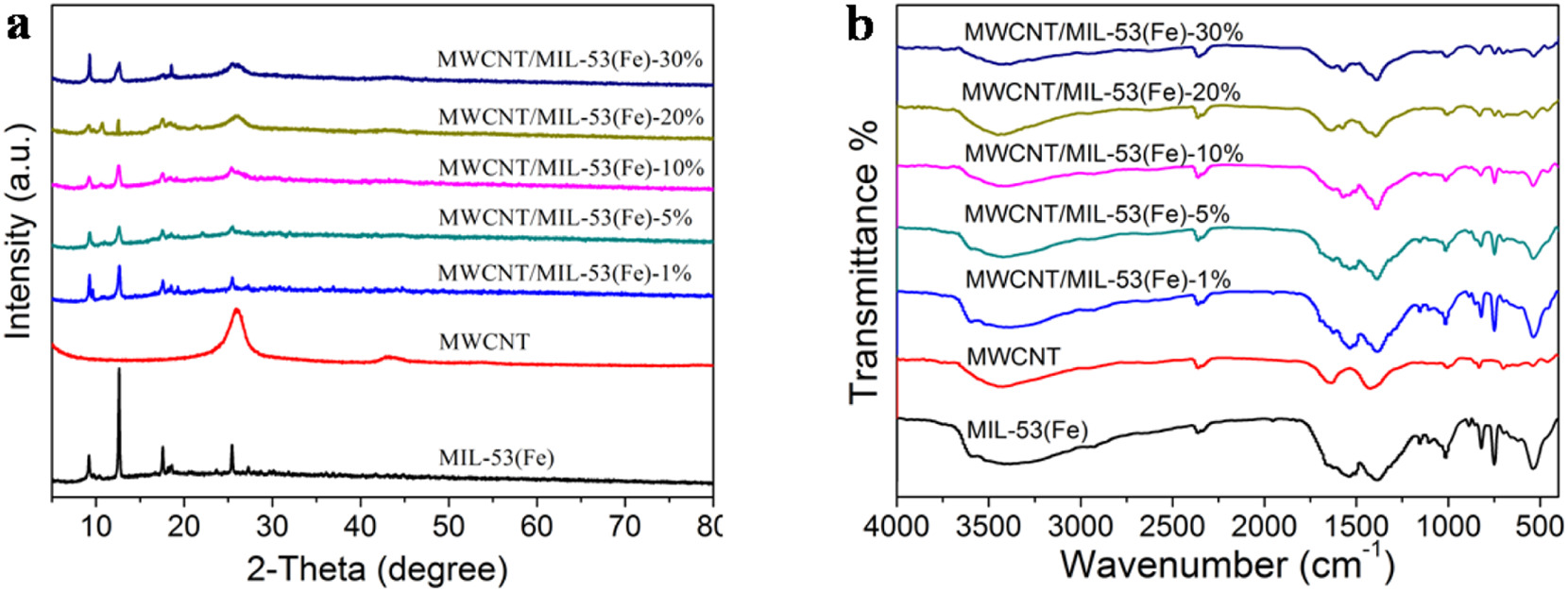
3) Other tests: XRD confirms the crystal structure of both components; FT-IR identifies functional groups like Fe-O and carboxyl; TGA shows enhanced thermal stability; XPS verifies elements C, O, Fe.
3. Application:
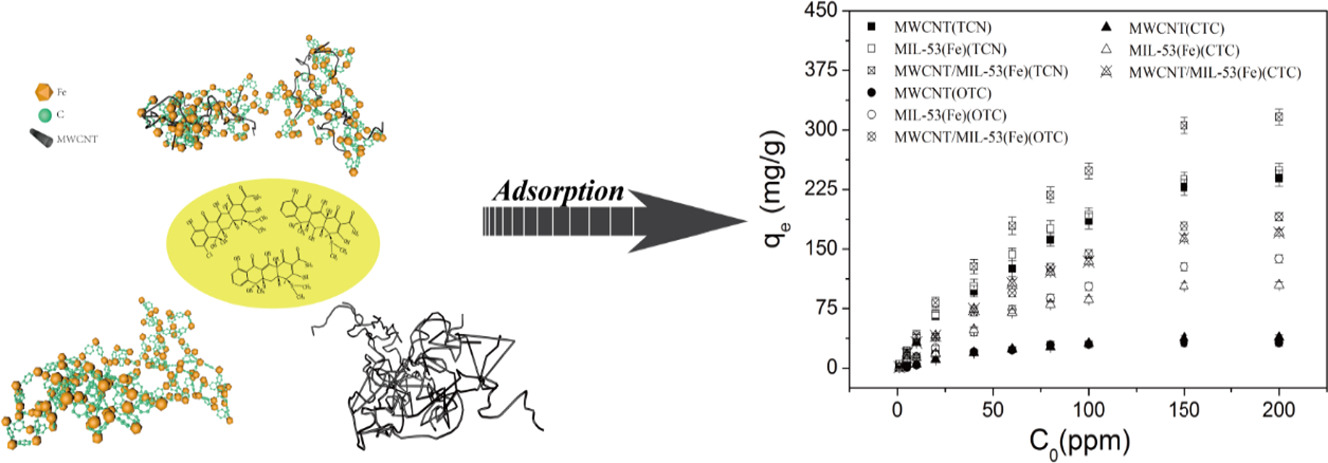
The material was tested for TCS adsorption. At 25°C and pH 7, maximum adsorption capacities for TCN, OTC, CTC are 364.37, 325.59, 180.68 mg/g, respectively, higher than MWCNT and MIL-53(Fe). It has good reusability (4 cycles) and water stability (low Fe leaching).
4. Mechanism:
Adsorption follows pseudo-second-order kinetics and Langmuir isotherms (homogeneous adsorption). Main mechanisms include π-π interactions between TCS benzene rings and the composite, pore/size-selective adsorption (smaller TCN molecules adsorb better), and weak electrostatic interactions.
Outlook:
This research demonstrates MWCNT/MIL-53(Fe) as a high-performance adsorbent for TCS removal, with improved stability and reusability, providing a new approach for water treatment of antibiotic pollutants.
Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent
Authors: Weiping Xiong, Guangming Zeng, Zhaohui Yang, Yaoyu Zhou, Chen Zhang, Min Cheng, Yang Liu, Liang Hu, Jia Wan, Chengyun Zhou, Rui Xu, Xin Li
DOI: 10.1016/j.scitotenv.2018.01.249
Link: https://www.sciencedirect.com/science/article/pii/S0048969718302912
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.


