Home >
News > Fluorescent Metal−Organic Framework MIL-53(Al) for Highly Selective and Sensitive Detection of Fe³⁺ in Aqueous Solution
Fluorescent Metal−Organic Framework MIL-53(Al) for Highly Selective and Sensitive Detection of Fe³⁺ in Aqueous Solution
Summary:
The authors from State Key Laboratory of Medicinal Chemical Biology (Nankai University), Synergetic Innovation Center of Chemical Science and Engineering (Tianjin), and Research Center for Analytical Sciences, College of Chemistry, Nankai University developed fluorescent metal-organic framework MIL-53(Al) with characteristics of good chemical stability, strong fluorescence, and specific cation exchange ability, achieving highly selective and sensitive detection of Fe³⁺ in aqueous solution and human urine samples.
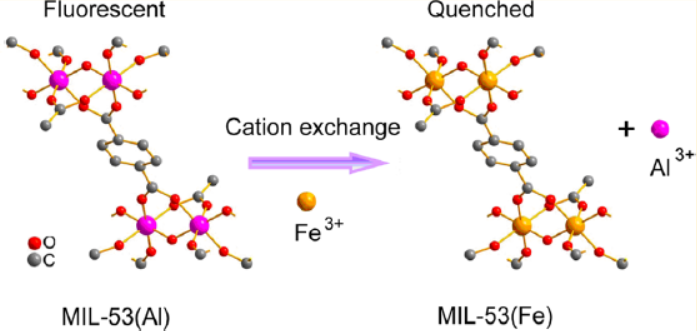
Background:
1. Fluorescent MOFs are widely used in sensing, but existing MOF-based metal ion detection mechanisms (such as metal-ligand coordination) have limited selectivity. Cation exchange between target ions and framework metal ions is a potential way to improve selectivity but has not been applied in sensing.
2. The authors proposed using MIL-53(Al) for Fe³⁺ detection based on cation exchange between Fe³⁺ and framework Al³⁺, realizing highly selective and sensitive detection with a detection limit of 0.9 μM.
Research Content:
1. Synthesis:
The authors synthesized MIL-53(Al) via a hydrothermal method. Al(NO₃)₃·9H₂O and 1,4-benzenedicarboxylic acid (BDC) were reacted in water at 220°C for 72 h, followed by purification with DMF and ethanol, and activation. MIL-53(Fe) was synthesized similarly using FeCl₃·6H₂O, BDC, DMF, and HF at 150°C for 72 h.
2. Characterizations:
1) Results of BET, pore size distribution: Not explicitly provided in the documents.
2) SEM/TEM tests show the particle size of the material: Not mentioned in the documents.
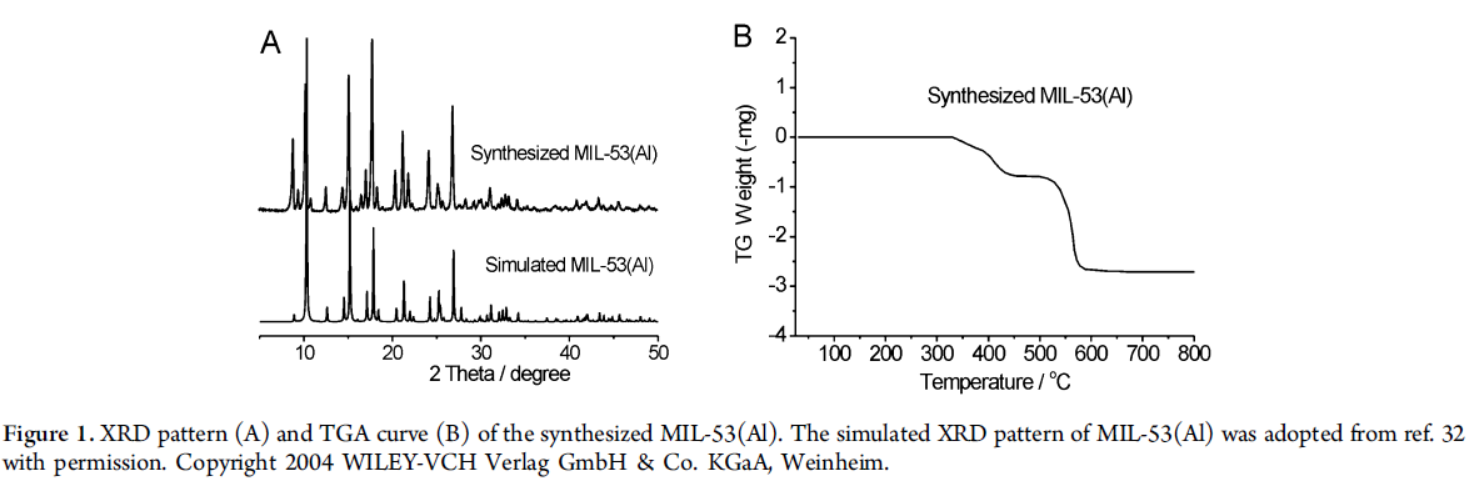
3) Other electrochemical or optical tests: XRD confirmed the crystal structure of MIL-53(Al) matched the simulated one; TGA showed it was stable up to 330°C; Fluorescence spectra revealed MIL-53(Al) had strong emission at 410 nm, while MIL-53(Fe) had weak emission; FT-IR and XPS verified the coordination environment.
3. Application:
The material was tested for Fe³⁺ detection. It showed a linear range of 3–200 μM, a detection limit of 0.9 μM, and high anti-interference. Spike recoveries in human urine samples were 98.2%–106.2%, consistent with ICPMS results.
4. Mechanism:
Fe³⁺ exchanges with framework Al³⁺ in MIL-53(Al), converting strong-fluorescent MIL-53(Al) to weak-fluorescent MIL-53(Fe), resulting in fluorescence quenching. This was confirmed by ICPMS (increased Al³⁺ in solution), XRD (structural transformation), and FT-IR (ligand coordination change).
Outlook:
This research develops a novel cation exchange-based fluorescent sensing method using MIL-53(Al) for Fe³⁺, providing a new strategy for designing selective MOF sensors, which is significant for environmental and biological sample analysis.
Fluorescent Metal−Organic Framework MIL-53(Al) for Highly Selective and Sensitive Detection of Fe³⁺ in Aqueous Solution
Authors: Cheng-Xiong Yang, Hu-Bo Ren, Xiu-Ping Yan
DOI: 10.1021/ac401387z
Link: https://pubs.acs.org/doi/10.1021/ac401387z
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

